GenoCTC
Core technology
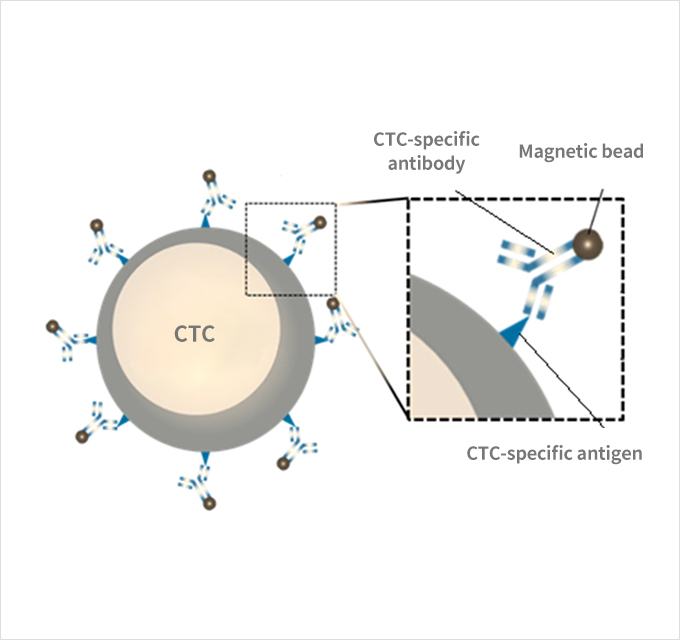
When the whole blood is mixed with reagents, the antibodies to which magnetic beads are bound and the antigen expressed
on the surface of cancer cells are specifically bound, and the surface of cancer cells is coated with magnetic nanobeads.
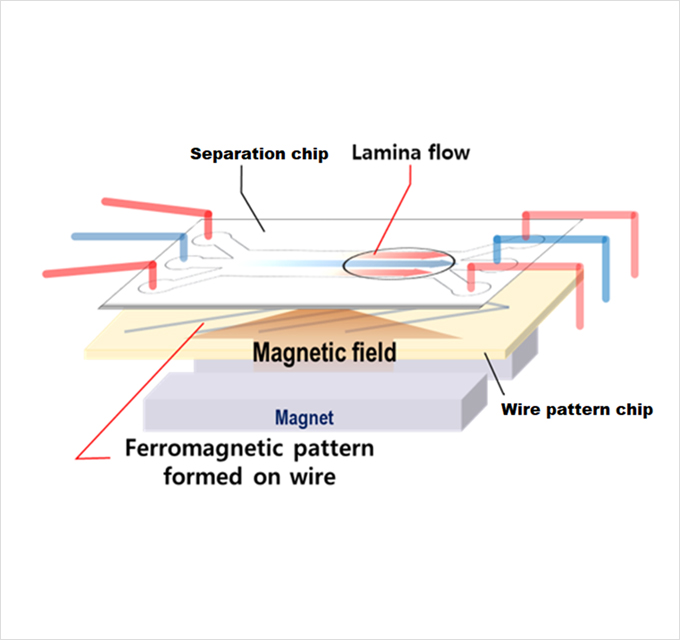
The GenoCTC device precisely controls the laminar flow within a microfluidic chip, preventing the blending of blood and buffer.
Cancer cells coated with magnetic beads, are specifically guided towards the buffer channel by the magnetized wire pattern chip, and separated specifically with minimal contamination of blood cells, yielding high purity CTCs.
Competitiveness
Innovative Medical Devices
- Precise and Reproducible
- Economical (Cost per test is reasonable)
- No cross-contamination among patient sample
- CTC enumeration and reporting within one day
- High separation purity allows immediate
downstream analysis

Engineered for Users
- Fully automated with the separation process is
monitored by sensors and camera assisted microscopes - The convenient device with a user-software interface
- Disposable cartridge prevents molecular
cross-contamination

Our platform
The GenoCTC is a fully automated device that allows the separation of circulating tumor cells (CTCs) from
blood samples, provided with disposable cartridges and reagent kits (EpCAMs) for CTC separation and the
GenoCTC Profiling Kit.
GenoCTC is a user-friendly device that does not require sample preparation, enabling sensitive, accurate
and high-purity isolation of CTCs from patient samples.
Performance
CTC isolation by GenoCTC involves microfluidic and immuno-magnetophoretic technology,
made feasible by our microfluidic separation chip, and ferromagnetic wire pattern chip.
GenoCTC allows isolation of CTCs based on cancer cell-specific biomarkers like EpCAM, MET, etc.
In the blood-based spiking test, we observe 80% recovery, 77% separation, and 90% purity.
Process
Sample collection
Peripheral blood sample
Binding with
antibody &
magnetic bead
GenoCTC Isolation kit™
Mounting onto
the cartridge

GenoCTC Cartridge™
Isolation on
GenoCTC

GenoCTC V5
CTC enumeration
characterization

GenoCTC Profiling kit™
Operation sequence
- Use 1 to 7.5 mL whole blood
- Use of cancer cell-specific (EMT, MET, etc.) biomarkers
- Use of targetable (c-MET) biomarkers

- User-friendly cartridge mounting
- Disposable cartridges prevent cross-infection
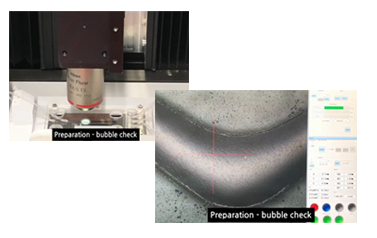
- Device preparation for CTC isolation
- Remove the bubble inside the separation chip and tubing
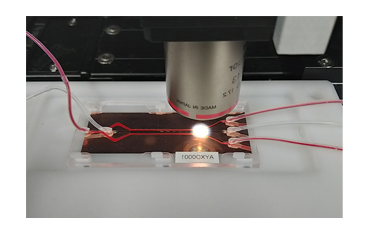
- Separation based on microfluidic and magnetophoresis
- High purity CTCs ready for downstream analysis.
- Fully Automated One-touch Separation
- ICC (Immunocytochemistry)
- Prognostic Analysis for Cancer
- Early detection of cancer recurrence
- Drug Reactivity and Resistance Response
- Genetic analysis (WGA, NGS, ddPCR, etc.)
Video
CTC Separation
GenoCTC

